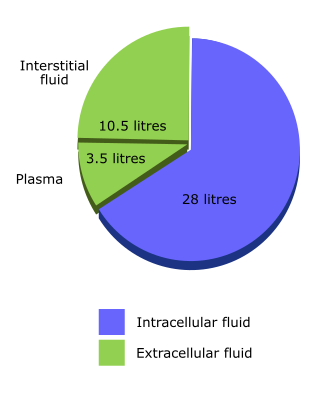
For isotonic solutions, which contain a mixture of ions and/or glucose, sodium content is the key determinant of the proportion of fluid that distributes to each compartment. If there is no sodium, the fluid distributes evenly throughout total body water, as with 5% glucose. If there is only sodium, plus its anion, fluid distributes only through the ECF.

For isotonic solutions, which contain a mixture of ions and/or glucose, sodium content is the key determinant of the proportion of fluid that distributes to each compartment. If there is no sodium, the fluid distributes evenly throughout total body water, as with 5% glucose. If there is only sodium, plus its anion, fluid distributes only through the ECF.
- 0.18% NaCl + 4% glucose
In 0.18% NaCl + 4% glucose, the glucose part distributes through total body water, whereas the NaCl part is entirely limited to the ECF.
1/5 is effectively 0.9% NaCl, and 4/5 5% glucose.
For every litre given, 200 ml stays in the ECF and 800 ml distributes evenly throughout total body water, i.e. 1/3 in ECF and 2/3 in ICF.
So 533 ml is in the ICF and 467 ml in the ECF, of which a quarter remains intravascular, i.e. 107 ml.
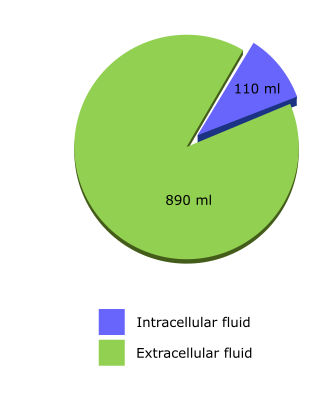
For isotonic solutions, which contain a mixture of ions and/or glucose, sodium content is the key determinant of the proportion of fluid that distributes to each compartment. If there is no sodium, the fluid distributes evenly throughout total body water, as with 5% glucose. If there is only sodium, plus its anion, fluid distributes only through the ECF.
- 0.18% NaCl + 4% glucose
- Hartmann's solution
For Hartmann's solution, sodium, chloride and calcium plus their associated water distribute only to the ECF, whereas lactate and potassium with their associated water can distribute through total body water.
So, for every litre given, a similar calculation shows that approximately 110 ml distributes to the ICF and 890 ml to the ECF.