Saturated Vapour Pressure
As a liquid continues to evaporate into a space above it, the pressure of its vapour increases. A point of equilibrium is reached at which the rate of evaporation equals that of condensation, when the space becomes saturated.
The pressure at this point is the saturated vapour pressure (SVP) (Fig 1).
Liquid molecules require energy to become vapour. This is the latent heat of vaporization. If this energy cannot be obtained from external sources then it is drawn from the liquid causing it to cool. This occurs in a vaporizer or when a nitrous oxide cylinder is used.
When a vapour condenses, some of this energy is returned as heat.
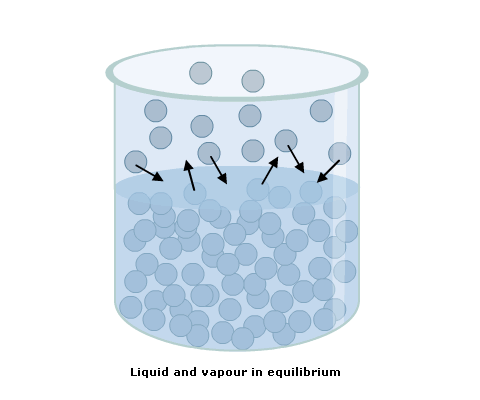
Fig 1 Saturated vapour pressure