Animation/Interaction loading...
Charles's Law
In 1787, Jacques Charles discovered that in a closed system with a fixed mass of gas, the volume is directly proportional to the absolute temperature provided the pressure remains constant, as shown:
Take the example of gas in a sealed cylinder, but with a moveable piston loaded to apply a constant pressure. If the cylinder is then heated, the pressure remains constant but the volume increases as the gas expands (Fig 3).
Select play to watch the animation.
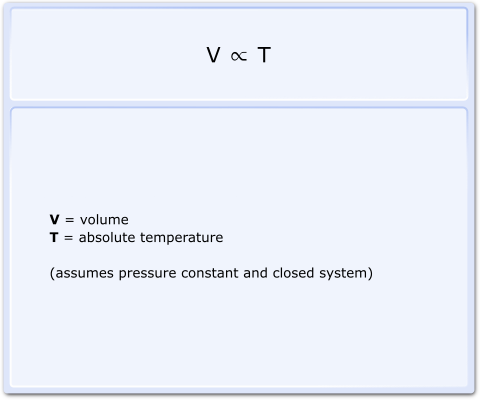
Fig 1 Charles’s Law equation
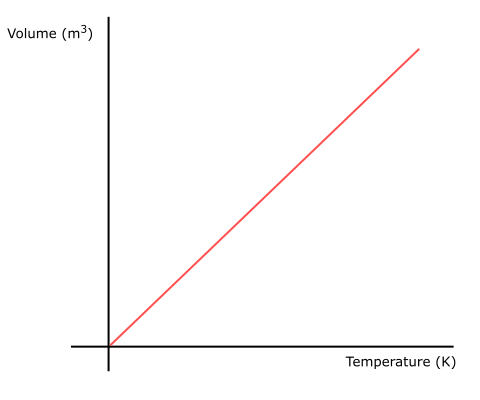
Fig 2 Charles’s Law graph
Fig 3 Heated gas expanding, increasing its volume