The laws of Boyle, Charles and Gay-Lussac are assimilated to produce the Combined Gas Law (Fig 1), which relates pressure, volume and temperature.
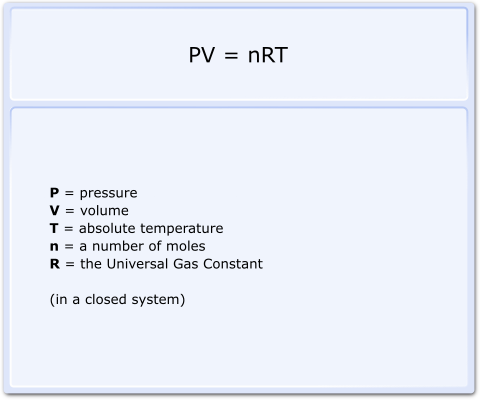
It is amended slightly, incorporating the number of molecules present to produce the Ideal Gas Law (Fig 2).
Changes in pressure, volume and temperature are therefore interdependent.
Practical application: A pressure gauge is used to measure the amount of gas in an oxygen cylinder. If the temperature and volume are constant then the pressure in the cylinder is directly related to the number of moles of gas in the cylinder and therefore its quantity.
Question: Would the same be true for a cylinder of nitrous oxide?
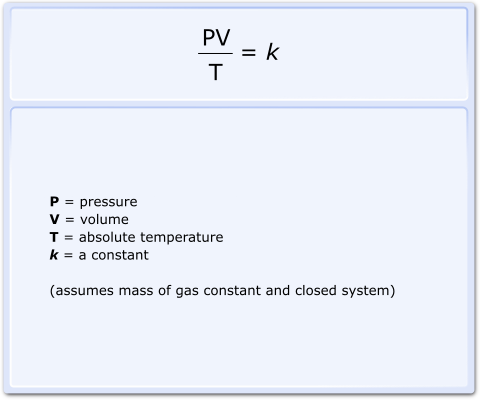
The laws of Boyle, Charles and Gay-Lussac are assimilated to produce the Combined Gas Law (Fig 1), which relates pressure, volume and temperature.
It is amended slightly, incorporating the number of molecules present to produce the Ideal Gas Law (Fig 2) .
Changes in pressure, volume and temperature are therefore interdependent.
Practical application: A pressure gauge is used to measure the amount of gas in an oxygen cylinder. If the temperature and volume are constant then the pressure in the cylinder is directly related to the number of moles of gas in the cylinder and therefore its quantity.
Question: Would the same be true for a cylinder of nitrous oxide?
The laws of Boyle, Charles and Gay-Lussac are assimilated to produce the Combined Gas Law (Fig 1), which relates pressure, volume and temperature.
It is amended slightly, incorporating the number of molecules present to produce the Ideal Gas Law (Fig 2).
Changes in pressure, volume and temperature are therefore interdependent.
Practical application: A pressure gauge is used to measure the amount of gas in an oxygen cylinder. If the temperature and volume are constant then the pressure in the cylinder is directly related to the number of moles of gas in the cylinder and therefore its quantity.
Question: Would the same be true for a cylinder of nitrous oxide?
Answer: No, because nitrous oxide has a critical temperature of 36.5°C, the cylinder contains liquid and vapour, so the gas laws do not apply. A cylinder of nitrous oxide has to be weighed to measure its contents.

The laws of Boyle, Charles and Gay-Lussac are assimilated to produce the Combined Gas Law (Fig 1), which relates pressure, volume and temperature.
It is amended slightly, incorporating the number of molecules present to produce the Ideal Gas Law (Fig 2).
Changes in pressure, volume and temperature are therefore interdependent.
Practical application: A pressure gauge is used to measure the amount of gas in an oxygen cylinder. If the temperature and volume are constant then the pressure in the cylinder is directly related to the number of moles of gas in the cylinder and therefore its quantity.
Question: Would the same be true for a cylinder of nitrous oxide?
Answer: No, because nitrous oxide has a critical temperature of 36.5°C, the cylinder contains liquid and vapour, so the gas laws do not apply. A cylinder of nitrous oxide has to be weighed to measure its contents.
