Introduction
When a gas is in contact with the liquid surface of another substance, molecules of the gas pass into the liquid and the gas is said to dissolve into the liquid. Molecules can also leave solution to return to the space above (Fig 1).
A gas moving in and out of solution behaves quite differently from a substance moving between its liquid and vapour phases, obeying Henry's Law.
As gas molecules dissolve, they exert an increasing partial pressure within the liquid. An equilibrium is eventually reached when there is no net movement of gas in or out of the liquid. At this point the partial pressure of the substance in solution equals its partial pressure in the gaseous phase above.
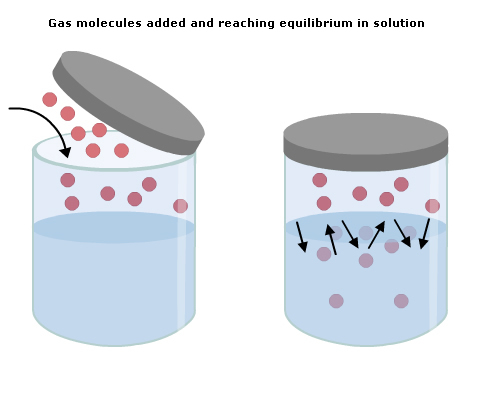
Fig 1 Solution of a gas in liquid