The movement of the molecules in a gas is random. This differs from other phases, such as in a solid where, though the molecules are in continuous motion, they oscillate about a mean position and are usually held in a regular formation (Fig 1).
The impact of gas molecules on a surface causes the pressure exerted. The kinetic energy is expressed as heat:
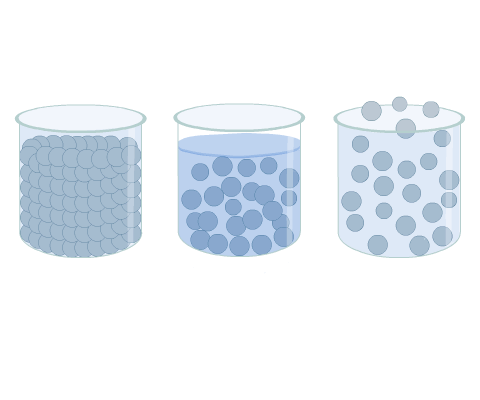
The movement of the molecules in a gas is random. This differs from other phases, such as in a solid where, though the molecules are in continuous motion, they oscillate about a mean position and are usually held in a regular formation (Fig 1).
The impact of gas molecules on a surface causes the pressure exerted. The kinetic energy is expressed as heat:
- Liquid phase
In the liquid phase more energy has been added to the molecules and the forces of attraction weaken. The molecules are freer to move throughout the liquid but are largely constrained within a boundary or surface.
The movement of the molecules in a gas is random. This differs from other phases, such as in a solid where, though the molecules are in continuous motion, they oscillate about a mean position and are usually held in a regular formation (Fig 1).
The impact of gas molecules on a surface causes the pressure exerted. The kinetic energy is expressed as heat:
- Liquid phase
- Liquid and vapour phase
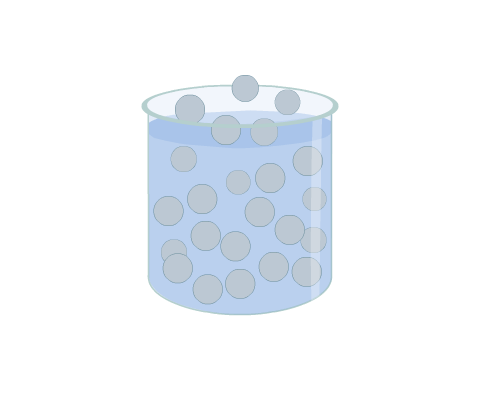
Molecules with sufficient kinetic energy escape from the surface, i.e. evaporate, and enter the gaseous state where they move randomly and are constrained only by the containing space. This gaseous state is considered to be a vapour while there is still liquid present. Movement of vapour molecules back into the liquid form, i.e. condensation, can also occur.
The movement of the molecules in a gas is random. This differs from other phases, such as in a solid where, though the molecules are in continuous motion, they oscillate about a mean position and are usually held in a regular formation (Fig 1).
The impact of gas molecules on a surface causes the pressure exerted. The kinetic energy is expressed as heat:
- Liquid phase
- Liquid and vapour phase
- Gas phase
When a substance reaches its critical temperature, the molecules have acquired so much kinetic energy that they cannot exist as a liquid and are no longer a vapour but a gas. Conversion back to a liquid can only be achieved by cooling.
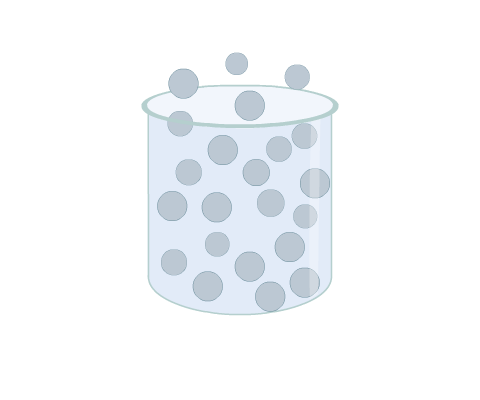
The movement of the molecules in a gas is random. This differs from other phases, such as in a solid where, though the molecules are in continuous motion, they oscillate about a mean position and are usually held in a regular formation (Fig 1).
The impact of gas molecules on a surface causes the pressure exerted. The kinetic energy is expressed as heat:
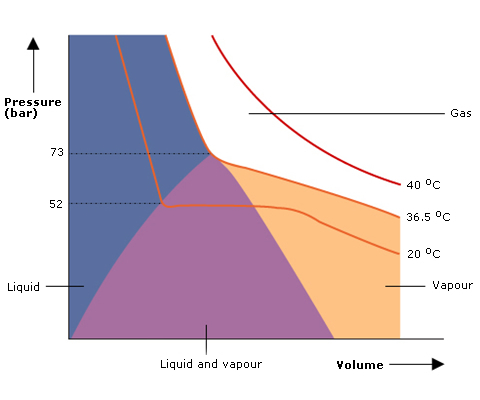
A practical example: Nitrous Oxide
The various phases in which nitrous oxide can exist at different temperatures are shown in Fig 5.
The critical temperature of nitrous oxide, i.e. the temperature above which it cannot be liquefied however much pressure is applied, is 36.5°C.
The curves of constant temperature are known as isotherms.