Animation/Interaction loading...
Boyle's Law
In 1662, Robert Boyle found that in a closed system with a fixed mass of gas, the pressure was inversely proportional to the volume, as shown:
Applying this to a cylinder of gas, if the piston is driven inwards to halve the volume then the pressure doubles (Fig 3).
Select play to watch the animation.
The relationship of P and V for a gas in two different settings is therefore:
P1V1 = P2V2
Each side of the equation represents one of these two settings.
For a gas stored under pressure, this formula can be used to calculate the volume available for use.
Example: A 5 L cylinder containing oxygen at a pressure of 100 atmospheres provides a supply of 500 L at 1 atmosphere.

Fig 1 Boyle’s Law equation
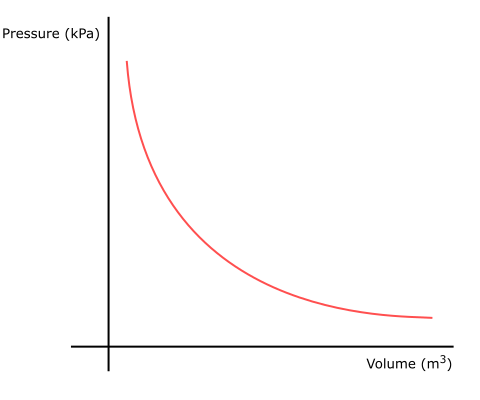
Fig 2 Boyle’s Law graph
Fig 3 A piston compressing gas.