Animation/Interaction loading...
Gay-Lussac's Law
In 1809, Joseph Gay-Lussac showed that if a fixed mass of gas is kept at a constant volume in a closed system, its pressure is directly proportional to the absolute temperature, as shown:
Again, take the example of gas in a sealed cylinder but with a constant volume. If heat is applied, the rise in pressure in the cylinder is directly proportional to the rise in absolute temperature (Fig 3).
Select play to watch the animation.
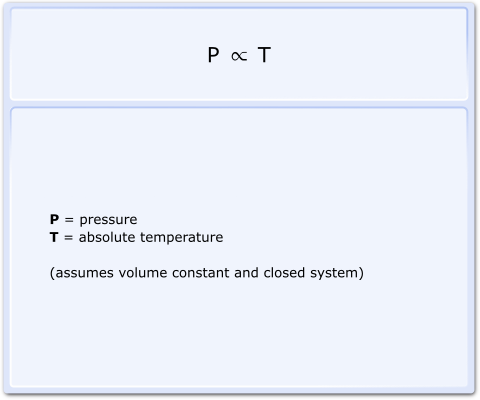
Fig 1 Gay-Lussac's Law equation
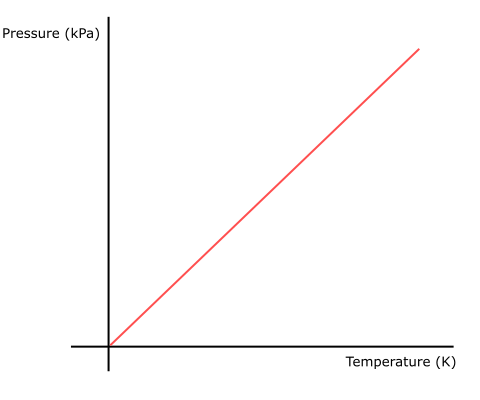
Fig 2 Gay-Lussac's Law graph
Fig 3 Heating gas in a confined space increases pressure