Questions
Question: What single variable changes the SVP of a substance?
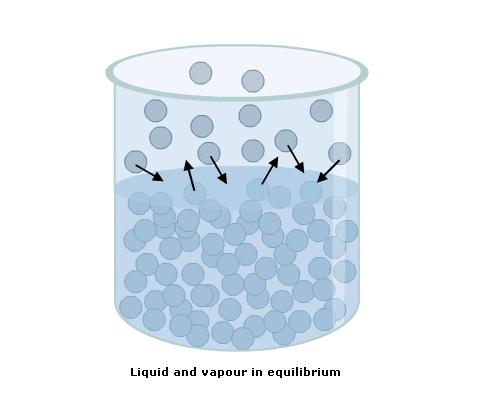
Question: What single variable changes the SVP of a substance?
Answer: The SVP increases progressively with a rise in temperature.
When saturated vapour is cooled, the SVP falls and some of the vapour condenses. This can be seen when exhaling on a cold day, and is used in simple condenser humidifiers, i.e. heat and moisture exchangers (HME).
Question: What happens when the SVP equals the atmospheric pressure?

Question: What single variable changes the SVP of a substance?
Answer: The SVP increases progressively with a rise in temperature.
When saturated vapour is cooled, the SVP falls and some of the vapour condenses. This can be seen when exhaling on a cold day, and is used in simple condenser humidifiers, i.e. heat and moisture exchangers (HME).
Question: What happens when the SVP equals the atmospheric pressure?
Answer: The liquid boils. The temperature at which the saturated vapour pressure equals the atmospheric presure is the boiling point.
