Solubility and Temperature
For different substances, the quantity of gas dissolving in a liquid at a given partial pressure can vary hugely and is related to their solubility coefficients.
This is sometimes described as a partition coefficient between two phases such as blood/gas, and describes the relative concentration in each phase when an agent has been distributed between them at equilibrium.
As the temperature increases, the molecules in the solution have more energy to get out of the liquid and so solubility falls (Fig 1).
Conversely, a fall in temperature increases the solubility of a gas in a liquid.
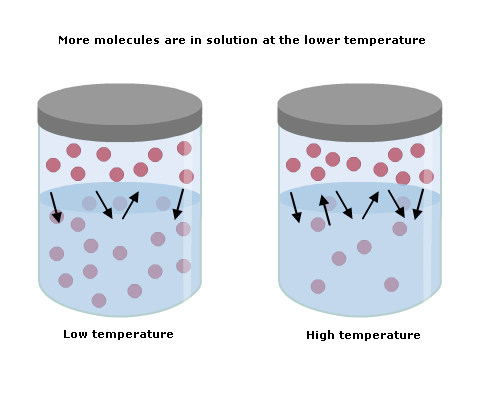
Fig 1 Effect of temperature on solubility